Table of Contents
- Introduction: Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs
- About Hikal Limited Panoli
- Open Positions and Job Details: Hikal Limited Panoli
- Job Location: Hikal Limited Panoli
- How to Apply: Hikal Limited Panoli
- Final Words: Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs
- Find More Jobs:-
Introduction: Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs
Welcome to MoneyJobs.in – We are excited to inform you about Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs For Quality Assurance Executive, Quality Assurance Assistant Manager, Quality Control Officer.
Hikal Limited is a well-established pharmaceutical and specialty chemical manufacturing company with a strong presence in regulated global markets. The company is known for its commitment to quality, compliance, innovation, and sustainable manufacturing practices. Hikal serves leading pharmaceutical, agrochemical, and specialty chemical customers across India and international markets.
Don’t miss this golden opportunity to join one of the leading names in the Chemical industry. Stay updated with detailed job descriptions, eligibility criteria, and interview schedules only on MoneyJobs.in – your next career move starts here!
About Hikal Limited Panoli
With a focus on cGMP compliance, robust quality systems, and advanced analytical capabilities, Hikal Limited provides an excellent working environment for professionals looking to build a long-term career in the pharmaceutical industry.
Manufacturing Units
Hikal Limited operates multiple state-of-the-art manufacturing facilities across India, including its advanced production unit located at Panoli GIDC, Gujarat.
Manufactures
- Active Pharmaceutical Ingredients (APIs)
- Specialty Chemicals
- Pharmaceutical Intermediates
Website
Open Positions and Job Details: Hikal Limited Panoli
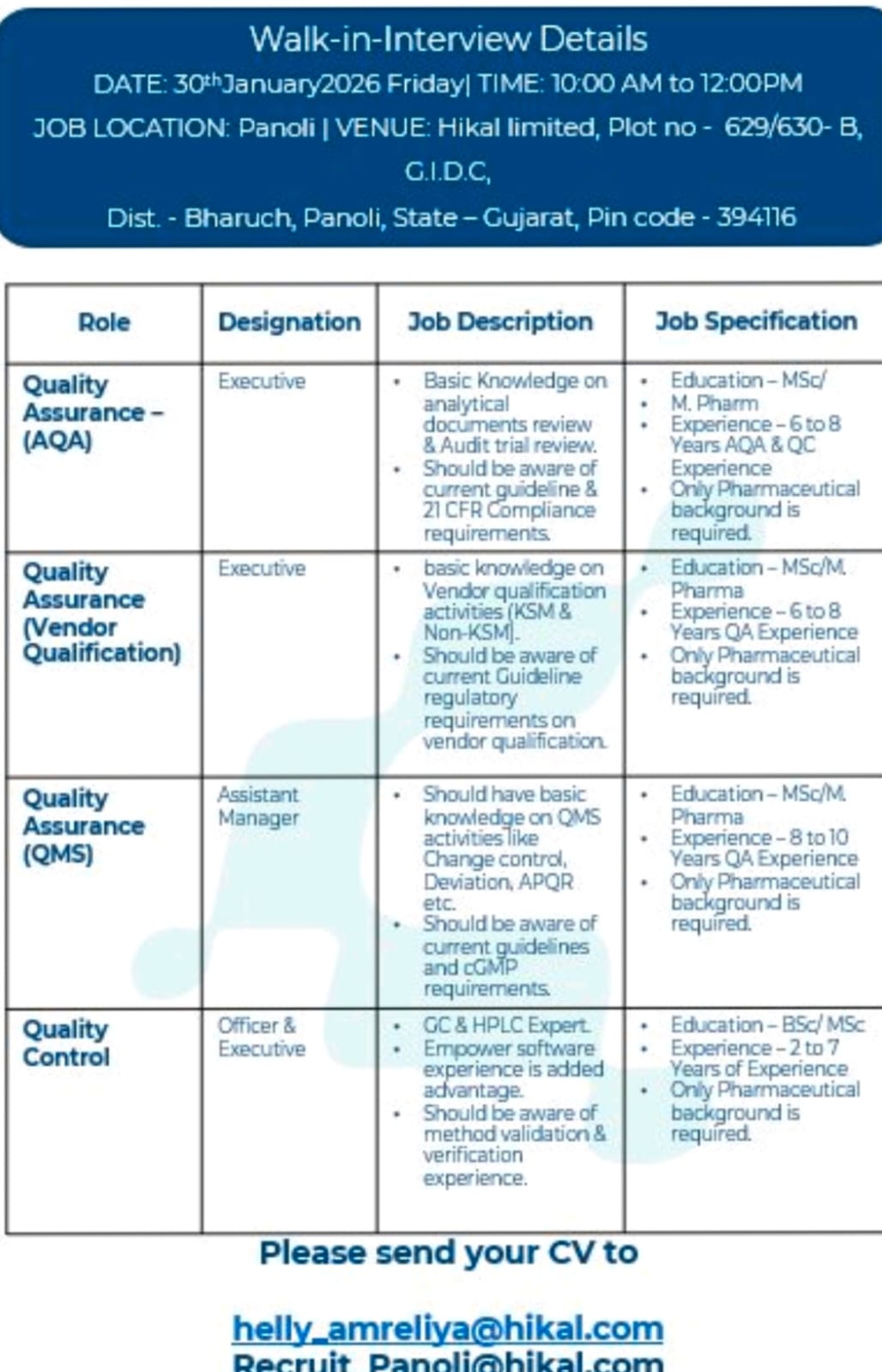
1. Position:- Quality Assurance Executive (AQA & Vendor Qualification)
Department: Quality Assurance
Total Requirement: As per company requirement
Qualification:-
MSc / M.Pharm (Only Pharmaceutical background required)
Years of Experience:-
6 to 8 years of relevant Quality Assurance experience
Responsibilities:-
- Review and management of analytical documents and audit trial records.
- Ensure compliance with current regulatory guidelines and documentation standards.
- Hands-on experience in Vendor Qualification activities including KSM and Non-KSM vendors.
- Knowledge of current regulatory requirements related to vendor qualification.
- Understanding of 21 CFR compliance and applicable quality guidelines.
- Support internal and external audits and ensure audit readiness at all times.
- Coordinate with cross-functional teams to maintain compliance and quality standards.
2. Position:- Quality Assurance Assistant Manager (QMS)
Department: Quality Assurance – Quality Management System
Total Requirement: As per company requirement
Qualification:-
MSc / M.Pharm (Only Pharmaceutical background required)
Years of Experience:-
8 to 10 years of Quality Assurance experience
Responsibilities:-
- Handling Quality Management System (QMS) activities including Change Control, Deviations, CAPA, and APQR.
- Ensure effective implementation and maintenance of QMS processes as per cGMP guidelines.
- Awareness and application of current regulatory guidelines and global compliance requirements.
- Coordination with different departments for investigations, risk assessments, and corrective actions.
- Preparation, review, and approval of quality-related documents and reports.
- Support regulatory inspections and customer audits with complete documentation and compliance readiness.
3. Position:- Quality Control Officer / Executive
Department: Quality Control
Total Requirement: As per company requirement
Qualification:-
BSc / MSc (Only Pharmaceutical background required)
Years of Experience:-
2 to 7 years of Quality Control experience
Responsibilities:-
Awareness of cGMP and laboratory regulatory requirements.
Expertise in Quality Control testing using analytical instruments such as HPLC and GC.
Experience in Empower software will be an added advantage.
Knowledge of method validation and method verification activities.
Routine analysis of raw materials, intermediates, and finished products.
Documentation and compliance with laboratory data integrity standards.
Job Location: Hikal Limited Panoli
Hikal Limited, Panoli GIDC, District Bharuch, Gujarat – 394116
How to Apply: Hikal Limited Panoli
Eligible and interested candidates are requested to attend the Walk-In Interview as per the details below:
Walk-In Interview Date: 30th January 2026 (Friday)
Time: 10:00 AM to 12:00 PM
Venue:
Hikal Limited
Plot No. 629/630-B, GIDC,
Panoli, Dist. Bharuch, Gujarat – 394116
Candidates who are unable to attend the walk-in interview may also send their updated CV to the following email IDs:
Final Words: Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs
This is an excellent opportunity for experienced Quality Assurance and Quality Control professionals to join a reputed pharmaceutical organization like Hikal Limited. Candidates with strong regulatory knowledge, hands-on experience in QA/QC operations, and a pharmaceutical background are highly encouraged to apply.
Stay connected with MoneyJobs.in for the latest updates on pharmaceutical walk-in interviews, QA/QC job openings, and career opportunities across India.
Your next career move starts here!
Find More Jobs:-
- Reliance Polyester Limited Recruitment 2026 | PSF Mechanical Engineer Jobs in Dahej, Bharuch
- Neelkanth Group of Companies Recruitment 2026 | Multiple Engineering & Technical Jobs in Gujarat
- DMCC Specialty Chemicals Limited Recruitment 2026 | QC Officer Jobs in Dahej
- GNFC Recruitment 2026 | Chief Manager, Data Scientist & Engineer Jobs at Gujarat PSU
- Deccan Fine Chemicals Walk-In Interview 2026 | Production & DCS Operations Jobs for Chemists
- Atul Ltd Ankleshwar Walk-In Interview 2026 | Production Jobs for BSc Chemistry Freshers & Experienced
- Zydus Lifesciences Walk-In Interview 2026 | Production, Packing & Engineering Jobs in Ahmedabad
- SRF Limited Dahej Walk-In Interview 2026 | Production, Instrumentation, Mechanical & QC Jobs
- Hikal Limited Panoli Walk-In Interview 2026 | QA & QC Executive and Assistant Manager Jobs
- Immediate Hiring at Deepak Foundation Khambhalia – Dwarka


















